A Sample of Published Research by Micki Ly M.D.
J Cutan Pathol 2010: 37: 282–286
doi: 10.1111/j.1600-0560.2009.01298.x
Journal of
Cutaneous Pathology
Molluscum contagiosum
involving an epidermoid cyst
with xanthogranuloma-like
reaction in an
HIV-infected patient
Bishr Aldabagh—Case Western Reserve School of Medicine, Cleveland, OH 44141, USA
Micki N Ly—Aloha Dermatology and Laser Center, Kahului, HI 96732, USA
Adam B Hessel—Buckeye Dermatology, Columbus, OH 44141, USA
Arif S. Usmani—Division of Dermatopathology, Bayless-Pathmark, Inc., Brecksville, OH 44141, USA
Background: Molluscum contagiosum (MC) causes characteristic cutaneous lesions that occur mainly in children, sexually active adults, and immunocompromised individuals, especially those with human immunodeficiency virus (HIV) infection. Patients infected with HIV, particularly those with advanced disease, have an increased incidence, up to 33.3%, of MC in non-anogenital areas. MC has been rarely found to be associated with epidermoid cysts.
Case report: A 44-year-old male with HIV infection presented with the complaint of a-3-month history of a tender nodule on the left neck. H&E stained sections showed a ruptured cyst, lined with squamous epithelium showing cytopathic changes of MC, and a xanthogranuloma-like inflammatory reaction with characteristic Touton-type giant cells.
Conclusion: MC infections are common, however MC associated with epidermoid cysts is infrequent. A few cases of MC occurring in epidermoid cysts have previously been reported. We are presenting a case of MC involving an epidermoid cyst in an AIDS patient, with a unique xanthogranuloma-like reaction. Xanthogranulomatous (XG) reactions have been infrequently reported in association with other viral infections, however, poxvirus-associated XG reaction has only been observed in animals. This is the first reported case of MC-associated XG reaction in humans.
Aldabagh B, Ly MN, Hessel AB, Usmani AS. Molluscum contagiosum involving an epidermoid cyst with xanthogranuloma-like reaction in an HIV-infected patient.
J Cutan Pathol 2010; 37: 282–286.
Introduction
Molluscum contagiosum (MC) is a cutaneous papular infectious disease caused by four closely related types of DNA poxviruses, MCV 1–4, and their variants.1 MC, originally described by Bateman,2 causes papular cutaneous lesions that mainly affect children, sexually active adults and immunocompromised individuals, especially those with HIV (human immunodeficiency virus) infection. Histologic features compatible with MC have been rarely reported in some epidermoid cysts.3–8 Patients infected with HIV-1 have an increased incidence of MC, and most have advanced disease with CD4 counts below 100 × 106/ L.9–12 There are rare reported cases of MC associated with folliculitis and epidermoid cyst in HIV patients.13,14 We are presenting, to our knowledge, the first case of MC in an epidermoid cyst with a peculiar xanthogranulomalike reaction in a patient with AIDS, which has not yet been reported in humans.
Case report: A 44-year-old male with HIV infection and CD4 count of 16 × 106/ L presented with a 3-month history of a tender nodule on the left neck. The lesion was an elevated firm inflamed subcutaneous nodule measuring 1.5 × 0.7 cm. The surface was erythematous, warm, and tender to palpation. However, there was no surface scale, punctum, or erosion. The clinical impression was an inflamed and ruptured epidermoid cyst, which was subsequently excised. The patient also had multiple white-topped umbilicated papules with size ranging from 3 to 1.8 cm on the neck, beard area, and left axilla. The patient had a history of squamous cell carcinoma and disseminated Mycobacterium avium infection, as well.
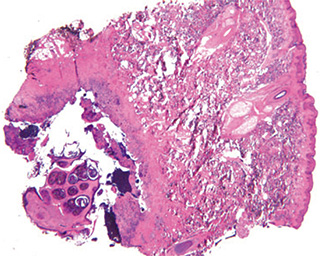
Histopathologic examination:
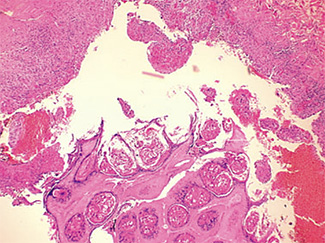
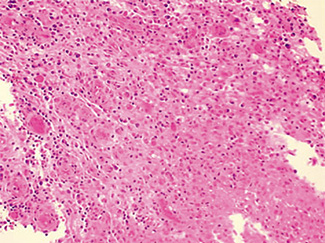
The excised specimen consisted of an elipse of skin and fragments of subcutaneous tissue. Hematoxylin & eosin (H&E) stained sections showed a cystic space in the deep dermis, the lining of which was mostly detached (Fig. 1). The intact portion of the cyst was lined by hyperplastic squamous epithelium which showed cytopathic changes, characteristic of MC (Fig. 2). The cells contained large red to blue cytoplasmic inclusions pushing the hyperchromatic and shrunken nuclei against the cell membrane (Fig. 3). There was associated dense granulomatous, suppurative, and chronic inflammation consistent with cyst rupture. Interestingly, there were collections of pale and foamy histiocytes with interspersed lymphocytes (Fig. 4) and occasional Touton-type giant cells giving the infiltrate a xanthogranulomalike appearance (Fig. 5). Occasional macrophages, containing amorphous material, were also noted which may represent engulfed viral particles (Fig. 6).
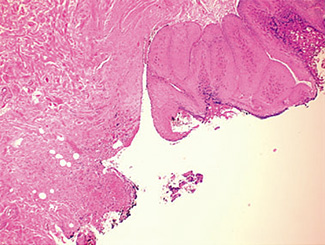
Discussion
Eleven cases of MC occurring in epidermoid cysts have been reported and are summarized in Table 1. In most of the cases, the HIV status of the patients was unknown. Smith et al.14 described one case of MC in an epidermoid cyst in an HIV patient. We are presenting another case of MC involving an epidermoid cyst in an AIDS patient, with a unique xanthogranuloma-like reaction. Poxvirus-associated xanthogranulomatous (XG) reactions have been observed in animals,15 however it has not yet been observed in humans.
MC often occurs in young children, sexually active adults and immunosuppressed persons, especially those with HIV infection. MC is caused by four closely related double stranded DNA-type of poxviruses, MCV 1–4 (MC virus), and their variants. MCV-1 infections are most common, and virtually all pediatric infections are caused by MCV-1.15,16 In patients infected with HIV,MCV-2 cause 60% of the infections,15,16 suggesting that HIV infection associated with molluscum does not represent recurrence of childhood molluscum.1 Almost 90% of adults have been shown to have antibodies to MC.17 However, clinical lesions are rarely observed in adults in nonanogenital areas when cellular immunity is intact. Molluscum is easily transmitted by direct skin-to-skin contact, especially if the skin is wet. Lesions are characteristically 3–5 mm smooth, firm, dome-shaped papules with a central umbilication. In children, lesions tend to be on the face, trunk, and extremities. In adults, molluscum is often sexually transmitted; and the distribution favors the lower abdomen, upper thigh, anus and in men, the penile shaft.1
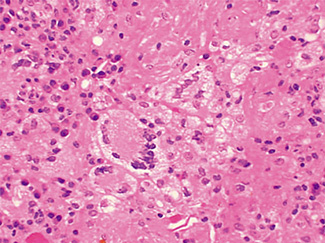
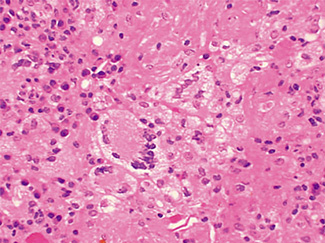
Patients infected with HIV have an increased incidence of MC, especially with advanced disease.9,10,18 Among persons infected with HIV, the prevalence of Fig. 5. Touton-type giant cells, typically seen in xanthogranuloma, are also noted. Fig. 6. A focus of granulomatous infiltrate with inclusion-like material in a macrophage. MC has been reported to range from 5 to 30%.1 A study by Koopman et al.19 on 72 patients with HIV reports the prevalence of MC lesions in patients with low CD4 counts (below 100 × 106/ L) to be as high as 33.3%. In another study of 27 patients with HIV infection, the mean CD4 count was 86 × 106/ L.12 There was also a statistically significant correlation between the CD4 count and extent of MC infection.12 In addition to a reduction in CD4 lymphocytes, a decrease in Langerhans cells in AIDS may also be a factor in the pathogenesis of MC.19 A lack of Langerhans cells is the characteristic of lesional skin of MC in non-HIV-infected persons.20,21
Table 1. Previous reports of MCV located in cysts
| Author | No. of patients | Age | Location of MCV in the cyst | HIV status |
|---|---|---|---|---|
| Hodge et al. (1973) 3 | 1 | 33 year old | Left thigh | Unknown |
| Fellner and Osowsky (1979)11 | 1 | 28 year old | Eyelid | Unknown |
| Henrick s et al. (1980)7 | 1 | 15 months old | Scalp | Unknown |
| Aloi (1985)4 | 1 | 6 year old | Left shoulder | Unknown |
| Ueyama et al. (1985)5 | 1 | 11 year old | Left lower eyelid | Unknown |
| Park et al. (1992)6 | 3 | 23, 31 and 23 year old | Penile shaft, inguinal, inguinal | Unknown |
| Egawa et al. (1995)8 | 1 | 68 year old | Neck, scalp, face, upper chest,upper back | Unknown |
| Smith et al. (1999)14 | 2 | 28 and 29 year old | Groin, Scrotum | (−), (+) |
| Our case (2001) | 1 | 44 year old | Left neck | (+) |
Table 2. Virus-associated granulomatous/xanthogranulomatous reactions (XG)
| Author | Patient | Description | Virus |
|---|---|---|---|
| Balfour et al. (1971)22 | 11-week-ol d infant | Juvenile xanthogranuloma | CMV |
| Nishimura et al. (1992)23 | 67-year-old man | Necrobiotic xanthogranuloma | HTLV-1 |
| Rodriguez Jurado et al. (2000)24 | 2-year-old girl | Necrobiotic xanthogranuloma | VZV |
| CMV = cytomegalovirus; HTLV-1 = human T-lymphotropic virus; VZV = varicella-zoster virus | |||
An interesting observation in our case was a tissue reaction similar to xanthogranuloma on microscopic examination in areas of epithelial denudation. This type of tissue reaction has not been described in association with epidermoid cyst rupture. A literature search revealed a few reports of virus-associated XG reactions. (Table 2).22– 24 Pinkus et al., in 1949, while studying inflammatory reaction to inoculated MC in humans noted a lymphohistiocytic reaction.25 Mehregan also described a granulomatous reaction in association with MC lesions in some of their 42 cases of MC.26 And finally, Niven et al. noted a granulomatous reaction in lesions of monkeys that were experimentally produced by poxvirus inoculation.15
XG reaction is not an uncommon inflammatory response and occurs in many organs and tissues. Cyst-associated XG reaction in extracutaneous sites have also been reported in the literature including several cases of colloid cysts of the third ventricle and cerebellar vermis,27– 30 urachus 31,32 and the subglottis.33 However none of these cases appear to be associated with the viral infection.
In the skin, it has been suggested that the preferred site of inoculation of MC virus is infundibular epithelium,34 which would explain cyst formation occasionally associated with MC lesions. Perhaps, the resulting infundibular epithelial hyperplasia following viral inoculation, in some case, occludes the infundibulum resulting in cystic dilation of the proximal portion of the hair follicle.
Herein, we describe a case of virus-associated XG reaction in a setting of MC-infected epidermoid cyst. In conventional MC infection, an inflammatory response is not induced because of localization of the virus to the epidermis. In our case, the dermal tissue was exposed to the viral particles because of the association with an epidermoid cyst and its subsequent rupture. The immunecompromised state of our patient may also have contributed to this peculiar inflammatory response.
References
- Odom RB, James WD, Berger TG eds. Andrew’s disease of the skin, 9th ed. Philadelphia, PA: W.B. Saunders Company, 2000; 501.
- Bateman F. Classics in clinical dermatology, 1st ed. Springfield, IL: Charles C. Thomas, 1953; 20.
- Hodge SJ, Fliegelman MT, Schrodt G, Owen LG. Molluscum congatiosum occurring in an epidermal cyst. Arch Dermatol 1973; 108: 257.
- Aloi FG, Pipione M. Molluscum congatiosum occurring in an epidermoid cyst. JCutan Pathol 1985; 12: 163.
- Ueyama Y, Osamura Y, Shimamura K, Nishimura M, Machida S, Tamaoki N. Molluscum contagiosum occurring in an epidermoid cyst on the eyelid. Acta Pathol Jpn 1985; 35: 193.
- Park SK, Lee JY,Kim YH,Kim S-Y, ChoBK, Houh W. Molluscum contagiosum occurring in an epidermal cyst: report of 3 cases. J Dermatol 1992; 19: 119.
- Henricks WF, Myer DE, Hu C-H. Molluscum contagiosum in an epidermal inclusion cyst. Cutis 1980; 26: 180.
- Egawa K, Honda Y, Ono T. Multiple giant molluscum contagiosa with cyst formation. Am J Dermatopathol 1995; 17: 414.
- Matis WL, Triana A, Shapiro R, Eldred L, Polk BF, Hood AF. Dermatologic findings associated with human immunodeficiency virus infection. J Am Acad Dermatol 1987; 17: 746.
- Katzman M, Carey JT, Elmets CA, Jacobs GH, Lederman MM. Molluscum contagiosum and the acquired immunodeficiency syndrome: clinical and immunological details of two cases. Br J Dermatol 1987; 116: 131
- Fellner MJ, Osowsky MJ. Molluscum contagiosum in an epidermal inclusion cyst on the eyelid. Int J Dermatol 1979; 18: 160.
- Schwartz JJ, Myskowski PL. Molluscum contagiosum in patients with human immunodeficiency virus infection: a review of twenty-seven patients. JAmAcadDermatol 1992; 27: 583.
- Weinberg JM, Mysliwiec A, Turiansky GW, Redfield R, James WD. Viral folliculitis typical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol 1999; 133: 983.
- Smith KJ, Yeager J, Skelton H. Molluscum contagiosum: its clinical, histopathologic, and immunohistochemical spectrum. Int J Dermatol 1999; 38: 664.
- Niven JS, Armstrong JA, Andrewes CH, Pereira HG, Valentine RC. Subcutaneous ‘growths’ in monkeys produced by a poxvirus. J Pathol Bacteriol 1961; 81: 1.
- Nelson JA, Ghazal P, Wiley CA. Role of opportunistic viral infections in AIDS. AIDS 1990; 4: 1.
- Scholz J, Rosen-Wolff A, Bugert J, et al. Molecular epidemiology of molluscum contagiosum. JInfectDis 1988; 158: 898.
- Postleth WR. Molluscum contagiosum: a review. Arch Environ Health 1970; 21: 432.
- Koopman RJJ, van Merrienboer FC, Vreden SG, Dolmans WM. et al. Molluscum contagiosum: a marker for advanced HIV infection. Br J Dermatol 1992; 126: 528.
- Betisto DV, Sanchez MR, Baer R, et al. Reduced langerhans cell Ia antigen and APT-ase activity in patients with acquired immunodeficiency syndrome. NEng J Med 1984; 310: 1279.
- Bhawan J, Dayal Y, Bhan AK. Langerhans cells in molluscum contagiosum, verruca vulgaris, plantar wart, and condyloma acuminatum. JAmAcadDermatol 1986; 15: 645.
- Balfour HH Jr, Speicher CE, McReynolds DG, Nesbit ME. Juvenile xanthogranuloma associated with cytomegalovirus infection. AmJMed 1971; 50: 380.
- Nishimura M, Takano-Nishimura Y, Yano I, Hayashi N, Toshitani S. Necrobiotic xanthogranuloma in a human T-lymphotropic virus type 1 carrier. J Am Acad Dermatol 1992; 27: 886.
- Rodriquez-Jurado R, Duran-McKinster C, Ruiz-Maldonado R. Benign cephalic histiocytosis progressing into juvenile xanthogranuloma. A non-langerhans cell histiocytosis transforming under the influence of a virus?. Am J Dermatopathol 2000; 22: 70.
- Pinkus H, Frisch H. Inflammatory reactions to molluscum contagiosum, possibly of immunologic nature. J Invest Dermatol 1949; 13: 289.
- Mehran AH. Molluscum contagiosum: a clinicopathologic study. Arch Dermatol 1961; 84: 123.
- Kudesia S, Das S, Shankar SK, Santosh V, Reddy AK. Colloid cyst xanthogranuloma of the third ventricle – a case report. Indian J Pathol Microbiol 1996; 39(3): 221.
- Tada M, Koiwa M, Chono Y, et al. Neuroepithelial (colloid) cyst of the cerebellar vermis containing a xanthogranuloma. Am J Neuroradiol 1993; 14(4): 951.
- Matsushima T, Fukui M, Kitamura K, Soejima T, Ohta M, Okano H. Mixed colloid cyst-xanthogranuloma of the third ventricle. A light and electron microscopic study. Surg Neurol 1985; 24(4): 457.
- Shuangshoti S, Phonprasert C, Suwanwela N, Netsky MG. Combined neuroepithelial (colloid) cyst and xanthogranuloma (xanthoma) in the third ventricle. Neurology 1975; 25(6): 547.
- Kinebuchi Y, Nakazawa M, Fujiwara M, Yoneyama T. Urachal xanthogranuloma caused by a swallowed fish bone: a case report. Hinyokika Kiyo 2001; 47(11): 797. (Review Japanese).
- Kuo TL, Cheng C. Xanthogranulomatous inflammation of urachus mimicking urachal carcinoma. Urology 2009; 73(2): 443.e13–14. E-pub 2008 Jun 24.
- Sahhar HS, Marra S, Shahid R, Akhter J. Juvenile xanthogranuloma: a rare cause of subglottic cyst and stenosis. Ear Nose Throat J. 2003; 82(9): 725.
- Uehara M, Danno K. Central pitting of molluscum contagiosum. JCutan Pathol 1980; 7(3): 149.
Revanesse® Versa™+
We believe that everyone is naturally beautiful, which is why we specialize in helping our clients enhance their natural features with Revanesse® dermal fillers. We take special care to provide subtle and natural-looking results using Revanesse® fillers so you can enhance your features without changing who you are. Book book a consultation today with Dr...Continue reading→
Advise from Maui Dermatologist Dr. Micki Ly M.D – in Her Own Words – “Mole removal can be a simple process”
Do You have a mole that is very obvious and unwanted? We can take care of that for you - it's called the mole removal. The process is very simple. To view more information on Mole Removal and the reasons that you should have Dr. Ly evaluate them, if you have any, please visit the...Continue reading→
Eyelid surgery (blepharoplasty), for a more youthful appearance, from the series “Advise from Maui Dermatologist Dr. Micki Ly M.D – in Her Own Words”
If you're having a little difficulty putting on eye shadow or your eyeliners run, you might be just needing an eye surgery. A simple procedure that will make you look younger and put the twinkle back in your eyes. To view more information on Upper Eye Lid Surgery, from Dr. Ly's website, please CLICK.Continue reading→